
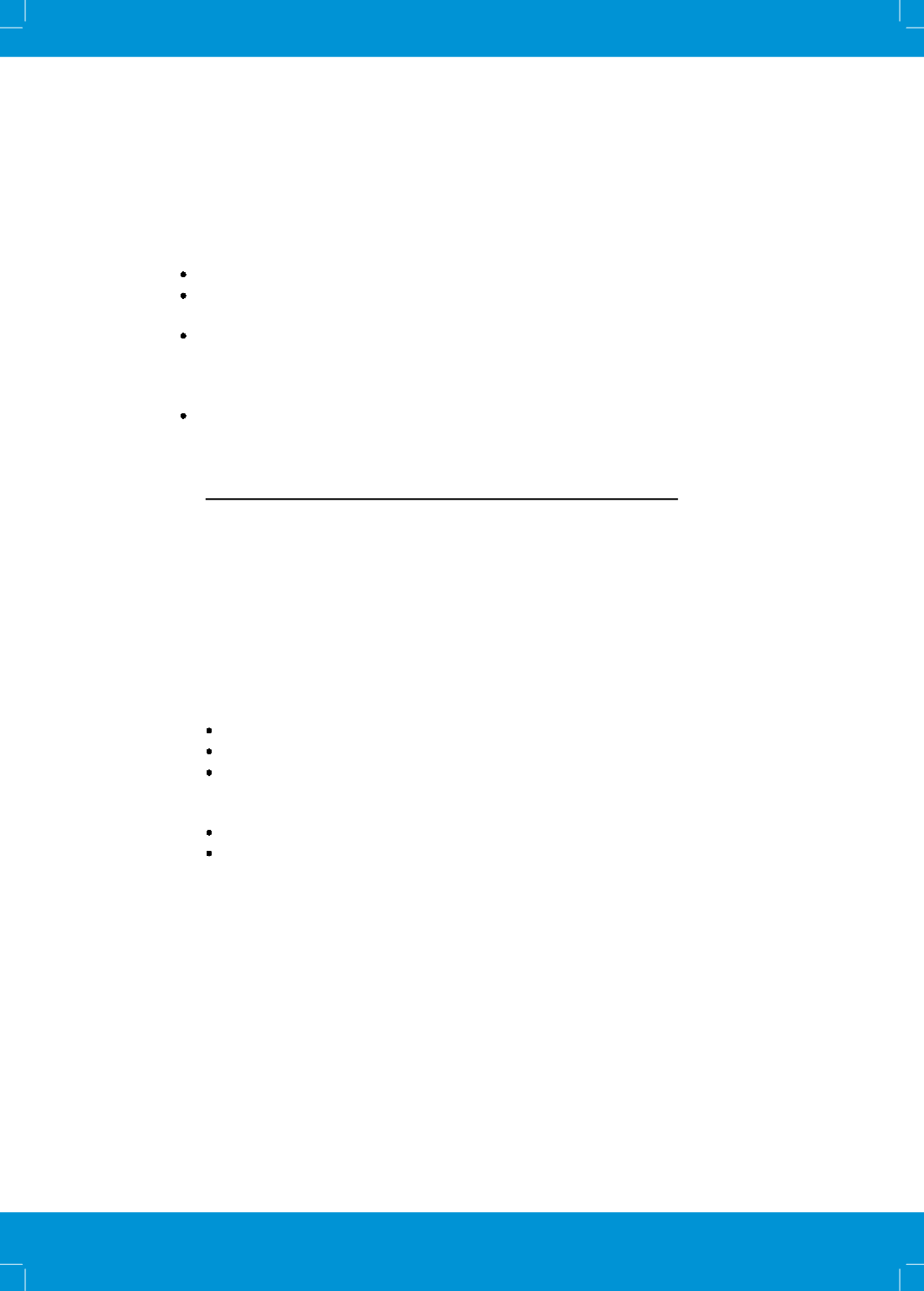
25% can be used but it may affect the viability of tubercle bacilli with prolonged
storage.
NOTE:
Samples for culture should
never be
collected in formalin.
If histo pathological examination is required, two samples should be
collected
No preservative should be used for any extra-pulmonary specimen for
culture. Necessary instructions are to be given to the concerned staff for
sending the biopsy specimen in normal saline for culture and NOT IN
FORMALIN as it will kill the bacilli.
Extra pulmonary specimens should never be collected or transported in
CPC.
3. TRANSPORTATION OF EXTRA PULMONARY SPECIMENS
As for pulmonary samples, extra pulmonary specimens will need to be
transported in cool boxes which maintain temperatures below 20
0
C for
specimens to be compatible for solid, liquid culture systems as well as molecular
methods. Triple packing system should be utilised for transportation. All
precautions that are followed for transporting pulmonary samples should be
followed. For sending material across international or state boundaries this
container may have to be packed in the same way with an additional outer
container; in such cases, special administrative arrangements with postal
authorities and/or airlines may be necessary.
When sending out specimens or when receiving them, check that:
Request forms are located separately from the specimen containers
Containers are labelled not on the cap but on the wall of the container
Each transport box has an accompanying list which identifies the
specimens and the patients; the information on the specimen
containers should correspond to that on the accompanying list.
Accompanying list contains the necessary data for each patient
Date of dispatch and particulars of the health centre are on the
accompanying list.
3.1 Specimens and request forms
All specimen transported to the laboratory must be accompanied by the request
form for C & DST in hard and soft copy formats (See C & DST request form). For
quality control reasons, the tests must be performed only upon written request of
authorized persons and oral requests without follow up written instructions should
not be allowed. It is also important that specimen request forms are kept
separate from the specimens themselves. Forms that have been contaminated
by specimens should be sterilized by autoclaving. If mistakes in filling request
forms and labelling of specimens are found, reject specimens and do not register
them. Document the arrival of specimens in the laboratory and note any delays in
143


















